Our question
While homologous recombination (HR) is the most accurate pathway to repair double-strand breaks (DSBs), misregulation of HR results in chromosomal rearrangements and aneuploidy. This occurs when ectopic (non-homologous) sequences, rather than sister chromatids or homologous chromosomes, are used as templates for repair. In heterochromatin, which occupies 30% of the genome, the risk of ectopic exchanges is extremely high because similar repeated sequences that can be used as templates for repair are present in non-homologous chromosomes. How do cells repair heterochromatic DSBs without generating massive genome instability?


Why is it important?
Aberrant recombination and chromosome rearrangements are involved in the etiology of cancer and other human disorders, and aging. Understanding the molecular mechanisms involved is expected to provide more effective tools for diagnosis and treatment of these diseases.

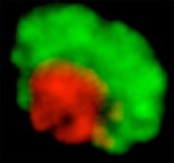
Figure 1. Left: Drosophila melanogaster. Right: Heterochromatic and euchromatic domains are clearly distinct in Drosophila cells.
Our approach
We work with the Drosophila model system (fruit fly), currently the best model for heterochromatin repair studies, and we combine genetic, biochemical and high-resolution imaging approaches. The organization of fly heterochromatin in a distinct domain is an exceptional advantage for cytological studies (Figure 1). To characterize repair progression, we express fluorescent-tagged repair components and follow their recruitment to foci at DSBs relative to the heterochromatin domain1.
What we discovered
Heterochromatin undergoes a global reorganization in response to damage: the entire domain expands and forms dynamic protrusions (Movie 1), while heterochromatic repair foci relocalize to the euchromatic space (Movie 2).
Movie 1: A Drosophila nucleus shows a compact heterochromatin domain before ionizing radiation (IR). The domain expands and forms dynamic protrusions after IR treatment, which induces DSBs.
Movie 2: A Drosophila nucleus shows early repair foci forming within the heterochromatin domain and relocalizing to outside the domain during repair.
DSBs are repaired by HR, but with significant differences from euchromatin. Repair starts within the domain (Movie 2), but can be completed only after relocalization of repair sites to the nuclear periphery2. This separation of repair steps in space and time requires SUMOylation and the Smc5/6 complex1,2, and is essential to prevent aberrant recombination and genome instability1. We suspect that this reorganization prevents ectopic exchanges by isolating the broken DNA and its homologous template from the main bulk of heterochromatic repeats during repair.
Current projects:
Our studies revealed the importance of nuclear dynamics in heterochromatic DSB repair, and raised new and exciting questions:
-
1)What mechanisms control these dynamics?
-
-
We employed genomic and proteomic approaches to identify the molecular components involved in the spatial and temporal regulation of heterochromatin repair, and we are now characterizing their function in the pathway.
2) How does deregulation of these processes affect genome stability in cancer cells and during aging? -
-
Deregulation of heterochromatin repair is potentially one of the most powerful driving forces for cancer and other genome-instability disorders, and a major cause for genome instability and cell lethality in old organisms. We are now investigating the consequences of inactivating heterochromatin repair pathways to genome stability and aging. We expect these studies to allow the development of better therapeutic approaches for cancer and other aging-related disorders.
-------------------------------------
References:
1 Chiolo I. et al. (2011) Double-strand breaks in heterochromatin move outside a dynamic HP1a domain to complete recombinational repair. Cell 144:732-44.
2 Ryu T. et al. (2015) Heterochromatic breaks move to the nuclear periphery to continue recombinational repair Nature Cell Biology 17:1401-11.
3 Caridi et al., Chiolo I. (2018). Nuclear F-actin and myosins drive relocalization of heterochromatin breaks. Nature, 559:35-7.
Our most recent studies 3 revealed that relocalization occurs through actin filaments assembled at repair sites (Movie 3). Myosins activated by Smc5/6 ride along these ‘highways’ for repair, enabling the directed motion of heterochromatic repair sites to the nuclear periphery.
Movie 3: A Drosophila nucleus shows repair foci sliding along nuclear actin filaments during relocalization to the nuclear periphery.